Organic Reaction
Starting from organic chemistry basic reactions we have Alkenes and how to prepare them well I am going to show you some simple shortcuts to remember these things.
Decarboxylation of mono-carboxylic Acids:
Hope you got it.
NUST Entry test MCQ regarding this topic is given below
The answer is C , methanoic acid .
"Decarboxylation of Benzoic acid will yield Benzene"
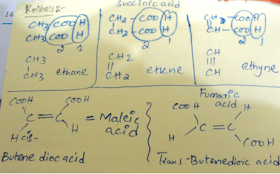
Kolbe’s electrolysis method is a difficult one , you must remember the products it yield when a certain acid is give.
This will help you remembering which acid yield what!
Maleic acid and Fumaric acid must be remembered.
Now Kolbe’s electrolytic method explained , try to write this down on your note book and then solve please you will understand.
All these methods are used to prepare alkanes :)
Clemnnsesn and Wolf-kishners method is used to prepare Alkenes by Reduction.
Starting from Acids.
Acetic acid > Aldehyde > primary alcohol > Ethane
Q: The Reduction of Aldehyde yield which type of alcohol the answer is P.Alcohol
Q: the reduction of ketones yields which type of alcohols well the answer is Secondary alcohol
Now getting to the alkenes production , just remember this shortcut.
Dehydrating agents
H2SO4 , P2O5 are dehydrating agents , mean de(remove) hydrate(water) so they remove water from alkenes forming alcohol. At different temperature they yield different alcohols. See book for that (IMPORTANT!!!)
Ozonolysis
See the picture below
Polymerization , production of benzene conversion , all covered in same topic :)
most important from board point of view. Distinguish 1-butyne from 2-butyne.
Wurtz synthesis
This reaction is used to prepare symmetrical alkane. This was question of 2013 board exam convert ethane into methane and methane into ethane :) I hope you can do it too now.
Different reactions used for preparing alky halides;
H------O------H -----------> HCl of left end SO2 in middle and HCl on right
Cl-----S-------Cl
||
O
Rest of reactions are same as above :)
that’s it from me :)
Entry # 8
By Mamoon Sharif











very helpful!
ReplyDeletekeeeep it up bro........ chaaaw gaye
ReplyDeleteawesome post ....very helpful and conceptual
ReplyDeletethank you :)
ReplyDeleteglad :)
ReplyDeletethank you bro :)
ReplyDeleteawesome !
ReplyDeletebohtt fit hai (y) keep it up :)
ReplyDeleteI Like your way of explanation (y)
ReplyDeleteNice work! These shortcuts will surely help me a lot :)
ReplyDeleteawlla bhae nice nd great work for studnts (y)
ReplyDeleteExellent job Sir thanks alot ...
ReplyDeletegreat!
ReplyDelete